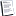
Ok, with the help of my human resources dept i was able to obtain a member handbook which shows the glossary of terms in the handbook. The following is uhc definition in my handbook of an experimental,investigational, and unproven service:
Medical,surgical,diagnostic,psychiatric,substance abuse or other healthcare services, technologies, supplies, treatments, procedures, drug therapies or devices that , at the time the company makes a determination regarding coverage in a particular case are dtermined to be:
* not approved by the u.s. Food and drug administration (fda) to be lawfully marketed for the proposed use and not identified in the american hospital formulary service, or the united states pharmacopoeia dispensing information, as appropriate for the use; or
* subject to review and approval by any institutional review board for the proposed use; or
* the subject of an ongoing clinical trial that meets the definition of a phase 1,2 or 3 clinical trial set forth in the fda regulations, regardless of whether the trial is actually subject to fda oversight; or
* not demonstrated through prevailing peer-reviewed medical literature to be safe and effective for treating or diagnosing the condition or illness for which its use is proposed.
As i have mentioned, i have had 2 appeals denied by uhc for adr surgery. They claim the surgery is an unproven service. I could really use some help. Thanks everyone gary

|